NOW APPROVED FOR CKD!
MAKE PROTECTION
YOUR SUPERPOWER
JARDIANCE® protects by reducing risk for
adult patients with CKD*1,2, HF†3,4 and T2D+CVD.‡5

CKD-Expanded
DID YOU KNOW THAT CKD ACTS AS A RISK MULTIPLIER?6,7
Cardio, renal, and metabolic diseases are interconnected!8
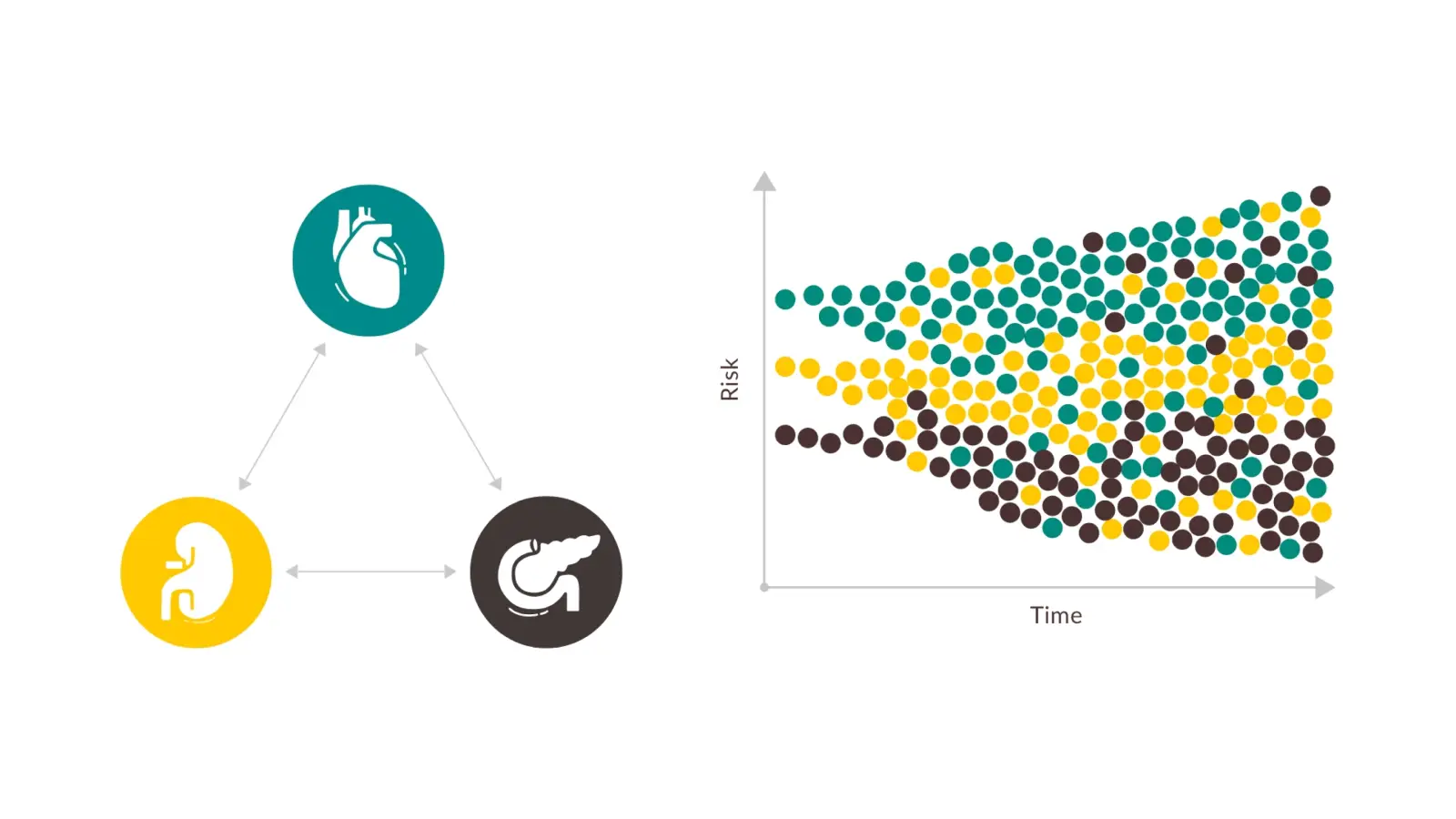

HELP PROTECT YOUR PATIENTS WITH JARDIANCE®
IN ADULT PATIENTS WITH CHRONIC KIDNEY DISEASE§
JARDIANCE® reduced kidney disease progression or risk of CV death*1,2

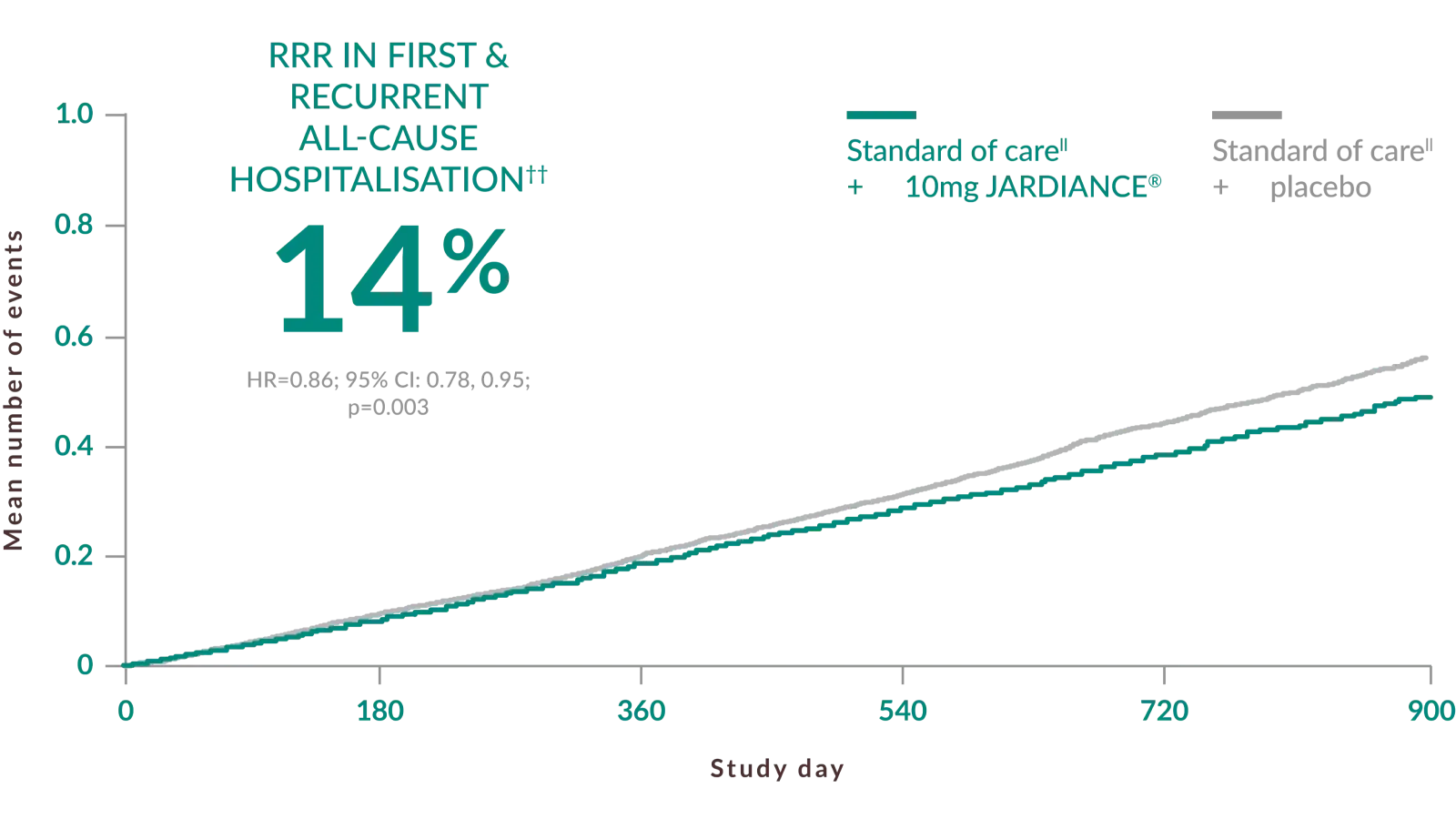
IN ADULT PATIENTS WITH CHRONIC KIDNEY DISEASE§
JARDIANCE® helped protect patients by giving more time out of hospital*††2

Prof. Will Herrington explains the key outcomes of the broadest SGLT2i CKD trial to date.
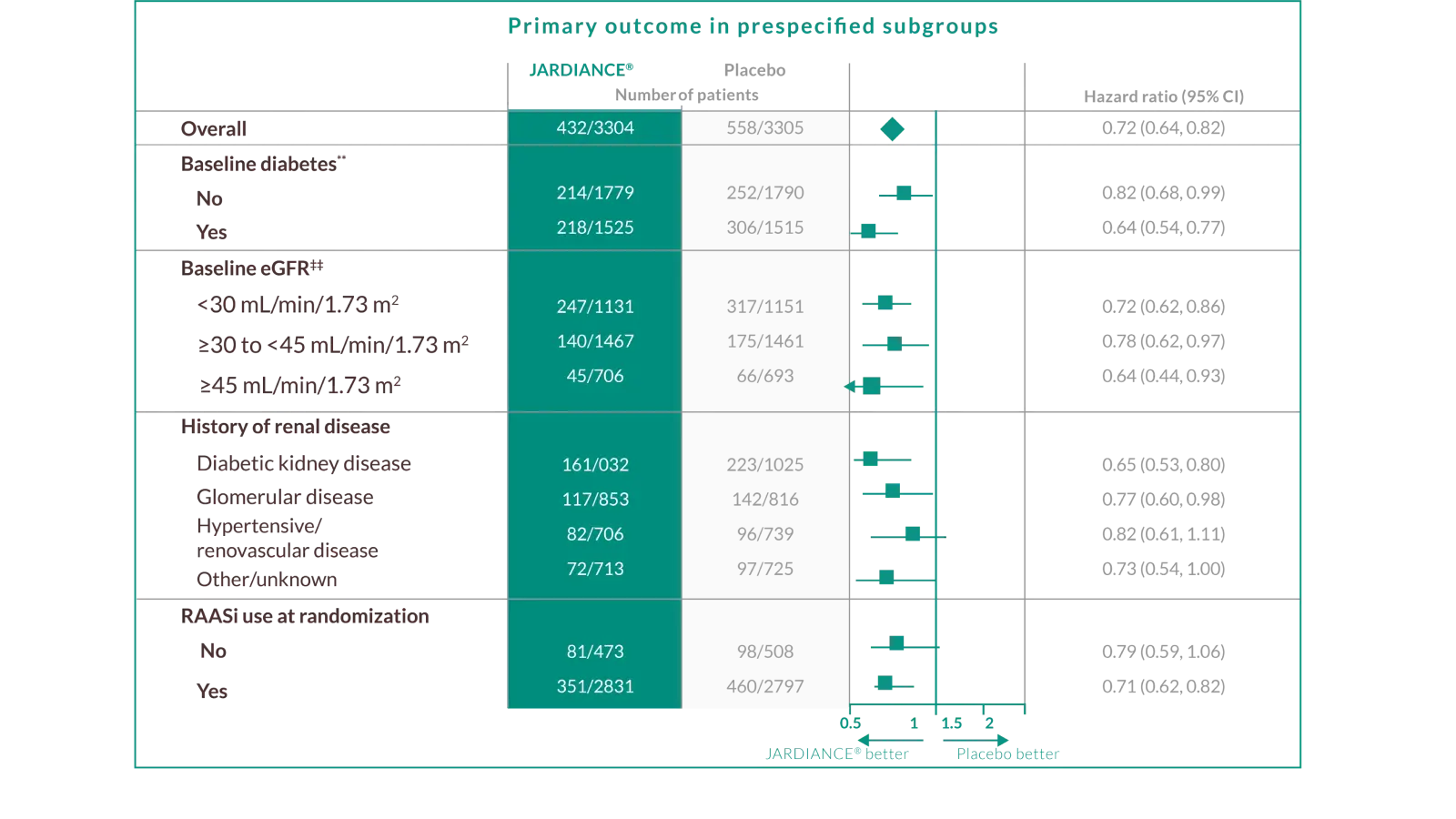
IN ADULT PATIENTS WITH CHRONIC KIDNEY DISEASE§
JARDIANCE® demonstrated consistent kidney and CV efficacy outcomes across the broadest spectrum of subgroups*2
JARDIANCE® is appropriate for a broad CKD patient population§ you see every day in your practice
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D
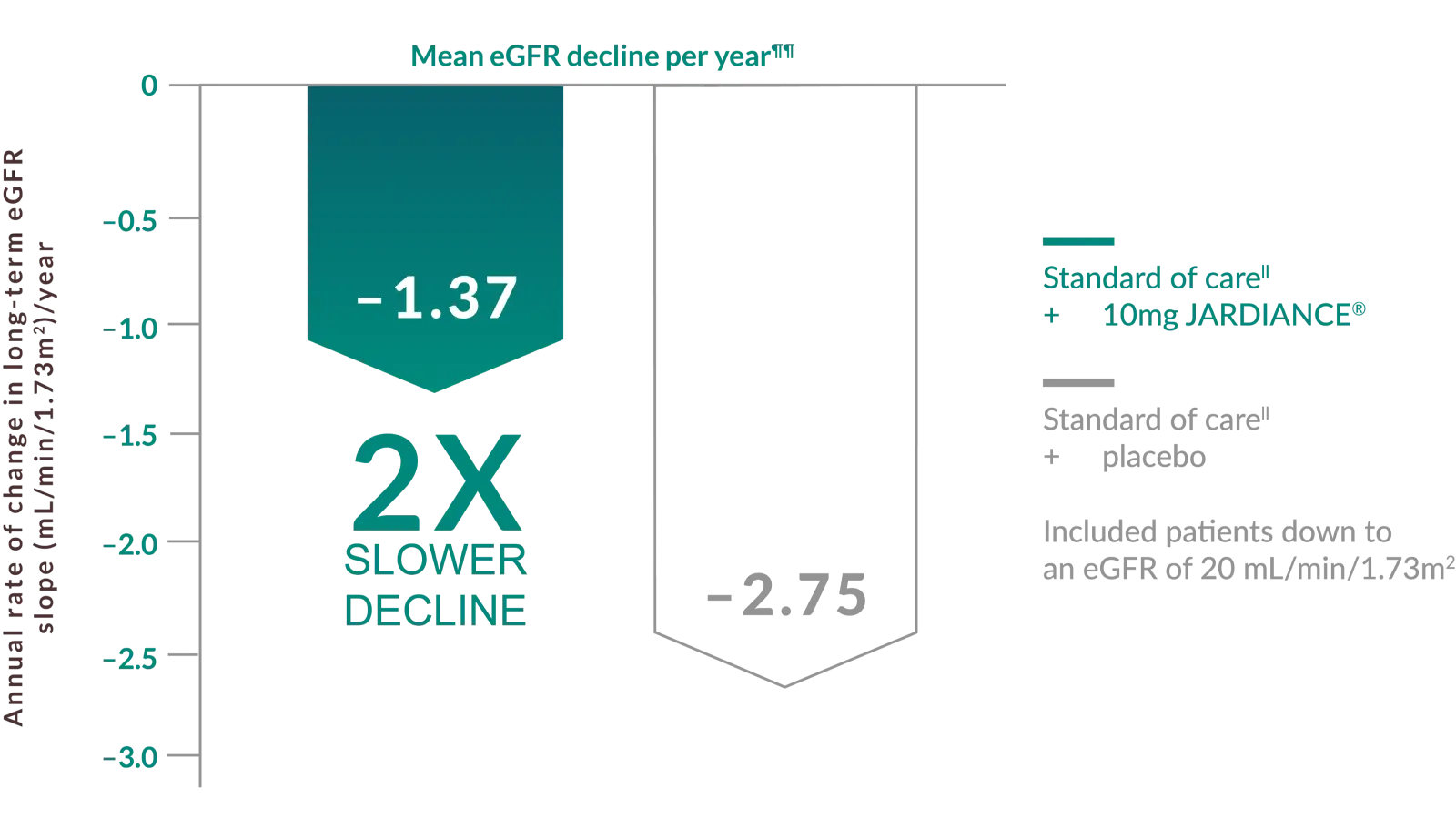
JARDIANCE® protected the kidney by slowing the decline of renal function over time*§§2
By slowing the decline, JARDIANCE® may prolong better kidney function vs standard of care##
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D
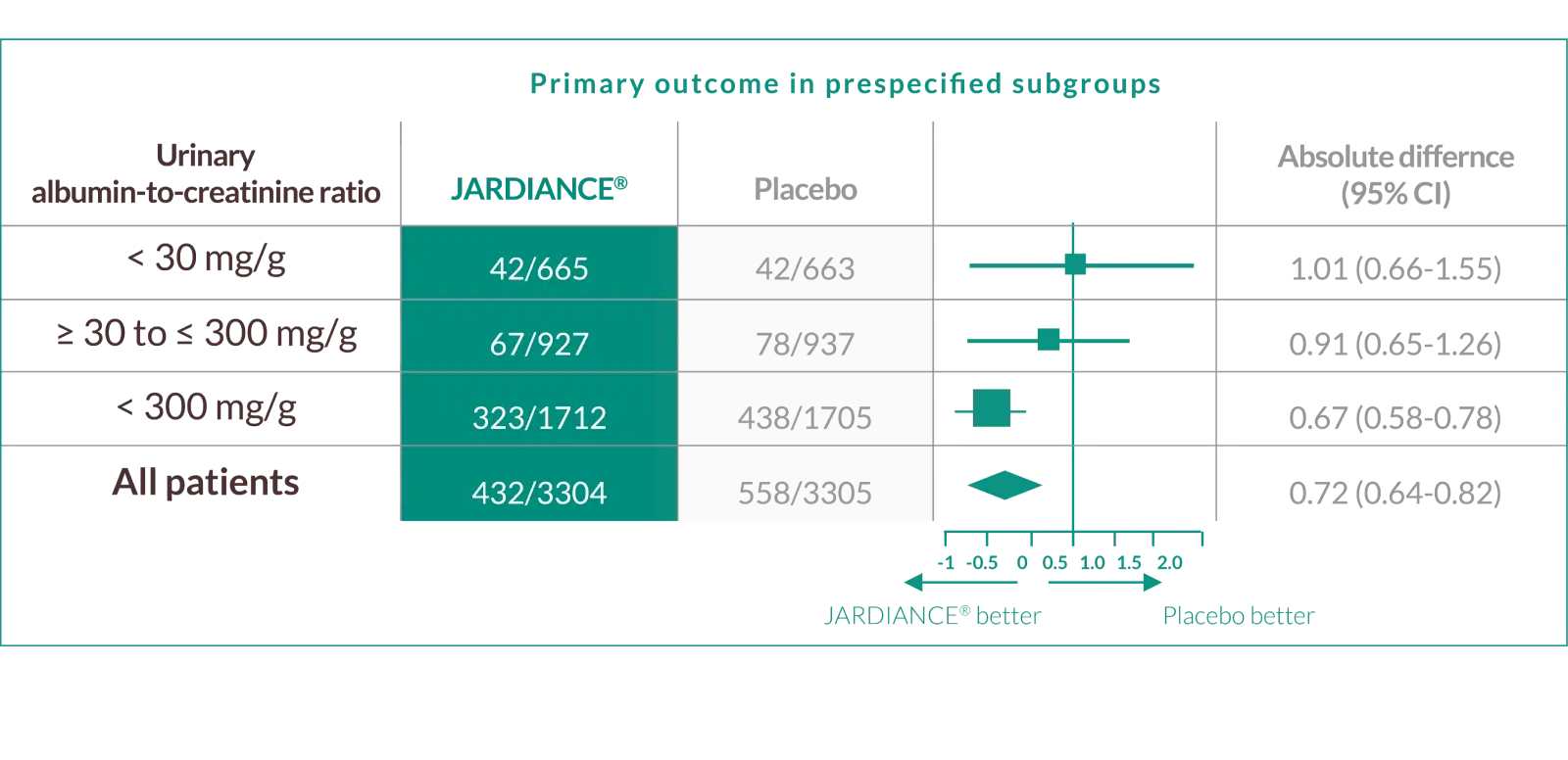
New evidence across the spectrum of uACR in a previously understudied*** patient population*2
In the primary endpoint, the benefit of JARDIANCE® was greater in patients with severely increased baseline albuminuria
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D
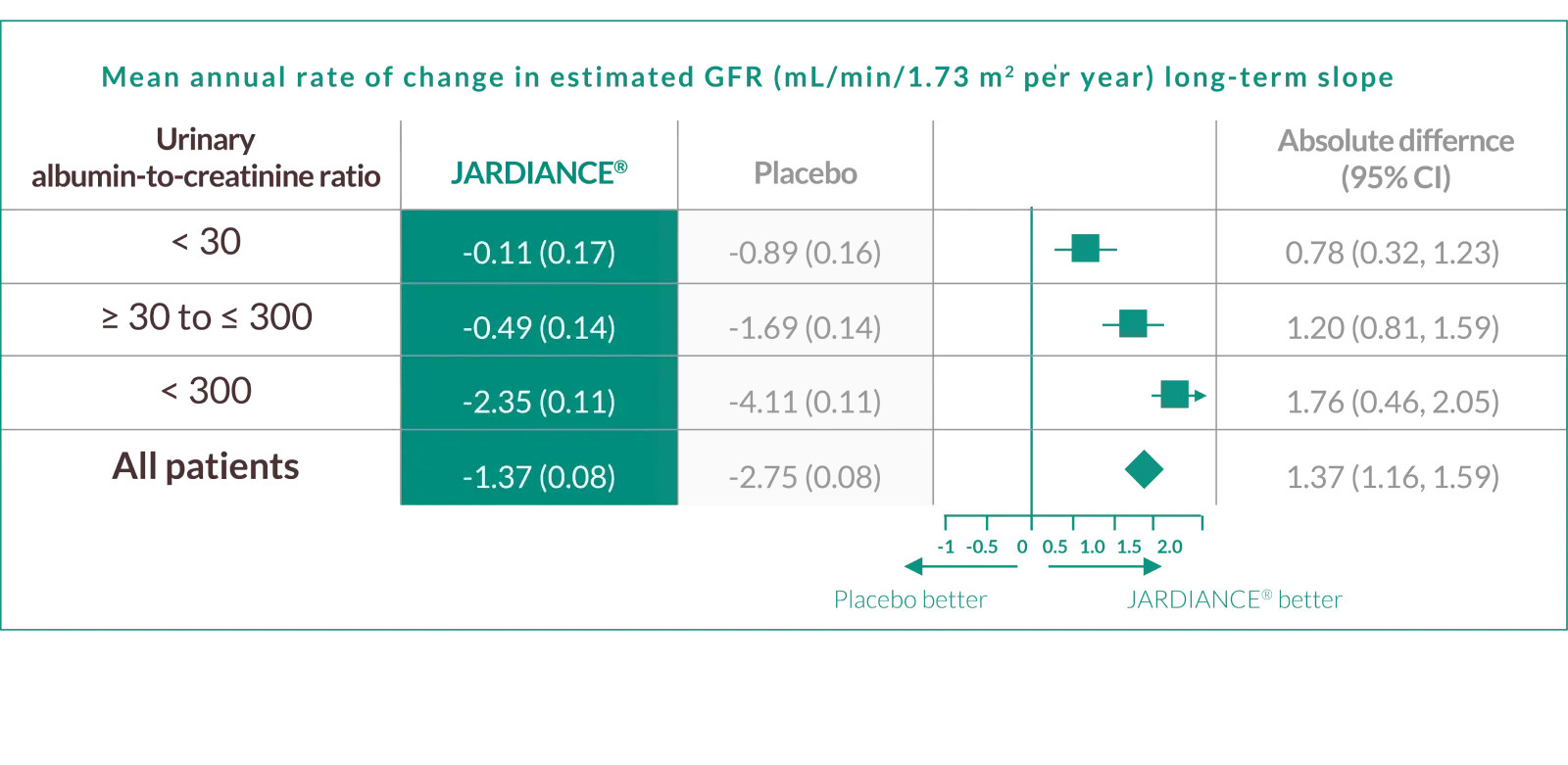
JARDIANCE® helped protect††† the kidneys across the spectrum of uACR by slowing eGFR decline
JARDIANCE® can protect††† kidney function by slowing eGFR decline even in patients with reduced eGFR§ who do not show kidney damage (indicated by uACR)
Related Content

GUIDELINE RECOMMENDATIONS

DOSING RECOMMENDATIONS

SAFETY & TOLERABILITY PROFILE
Indication & Footnotes
JARDIANCE® is indicated for the treatment of adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
as monotherapy when metformin is considered inappropriate due to intolerance
in addition to other medicinal products for the treatment of diabetes
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.
-
*
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety profile of JARDIANCE® 10 mg (n=3304) was evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease defined as end-stage kidney disease (the initiation of maintenance dialysis or receipt of a kidney transplant), a sustained decrease in the eGFR to <10 ml/min/1.73 m2, a sustained decrease in eGFR of ≥40% from baseline, or death from renal causes. Patients treated with JARDIANCE® experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
‡
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety of JARDIANCE® 10 mg (n=1863) were evaluated vs placebo (n=1867). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001). In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety of JARDIANCE 10 mg (n=2997) were evaluated vs placebo (n=2991). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).4,5
-
†
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety profile of JARDIANCE® 10 mg (n=1863) was evaluated vs placebo (n=1867). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001). In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety profile of JARDIANCE® 10 mg (n=2997) was evaluated vs placebo (n=2991). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).3,4
-
‡
The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE, composed of death from CV causes, nonfatal MI, or nonfatal stroke, as analyzed in the pooled JARDIANCE® group vs the placebo group. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. The 14% RRR in 3-point MACE (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) was driven by a reduction in the risk of CV death (HR=0.62; 95% CI: 0.49, 0.77).5
-
§
Adult patients with an eGFR ≥ 20, < 45 mL/min/1.73 m2; or an eGFR ≥ 45, < 90 mL/min/1.73 m2 with a uACR ≥ 200 mg/g.2
-
||
Standard of care: All patients received a RAASi unless an investigator judged that a RAASi was not indicated or tolerated.2
-
#
ARR for the primary composite outcome of kidney disease progression or CV death is 3.6% per patient-year at risk. Figure adapted from Herrington et al.2
-
¶
NNT: 28 (95% CI: 19, 53) per 2 years at risk.2
-
**
History of diabetes was defined as a patient-reported history of diabetes of any type, use of glucose-lowering medication, or a glycated hemoglobin level of at least 48 mmol per mole (6.5%) at the randomization visit. More than 95% of patients with diabetes had type 2.2
-
††
Hospitalization for any cause was a key secondary outcome of the EMPA-KIDNEY trial. The analysis of hospitalizations for any cause included the first and all subsequent events (JARDIANCE®, 1611 hospitalizations in 960 patients; placebo, 1895 hospitalizations in 1035 patients).2
-
‡‡
Values represent the measurement recorded at the randomization visit or the most recent local laboratory result recorded before randomisation.2
-
§§
Progression of kidney disease was a prespecified secondary outcome of the EMPA-KIDNEY trial.2
-
##
JARDIANCE® gives you the opportunity to delay the initiation of dialysis for patients with CKD2
-
¶¶
Values represent the mean (± SE) changes from 2 months after the first dose of JARDIANCE® or placebo to the final follow-up visit.2
-
***
Patients enrolled in the EMPA-KIDNEY trial included groups excluded from or under-represented in the other SGLT2i trials with a primary focus on kidney disease progression.2
-
†††
Annual rate of change in eGFR was a prespecified exploratory outcome in the EMPA-KIDNEY trial. Shown are the results of long-term slope, representing the mean (±SE) changes from 2 months after the first dose of JARDIANCE® or placebo to the final follow-up visit.1,2
-
ARR=absolute risk reduction; CAD=coronary artery disease; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; HF=heart failure; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HR=hazard ratio; LVEF=left ventricular ejection fraction; MACE=major adverse cardiovascular events; MI=myocardial infarction; NNT=number needed to treat; NYHA=New York Heart Association; PAD=peripheral artery disease; RAASi=renin-angiotensin-aldosterone system inhibitor; RRR=relative risk reduction; T2D=type 2 diabetes; uACR=urinary albumin-to-creatinine ratio.
References
-
JARDIANCE® [Local summary of product characteristics]. Ingelheim am Rhein, Germany.
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Packer M, Anker SD, Butler J, et al. EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
-
Jankowski J, Floege J, Fliser D, Böhm N, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157-1172.
-
Kalra S, Aydin H, Sahay M, et al. Cardiorenal syndrome in type 2 diabetes mellitus—rational use of sodium-glucose cotransporter-2 inhibitors. Eur Endocrinol. 2020;16(2):113-121.
-
McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
-
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
-
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Acad Cardiol. 2022;79(17):e263-e421.
-
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1):1-150.
Document ID: PC-SA-102620 | Expiry Date: 01/30/2027
